Clinical Indications
▶ Main conditions treated with botulinum toxin:
- Cervical dystonia (spasmodic torticollis) (a neuromuscular disorder involving the head and neck)
- Blepharospasm (excessive blinking)
- Severe primary axillary hyperhidrosis (excessive sweating)
- Strabismus (Squints)
- Achalasia (failure of the lower oesophageal sphincter to relax)
- Local intradermal injection of BTX-A is helpful in chronic focal neuropathies. The analgesic effects are not dependent on changes in muscle tone.
- Migraine and other headache disorders, although the evidence is conflicting in this indication
- Excessive sweating is a condition for the treatment of which FDA has approved the use of Botox.
▶ Off label uses:
- Idiopathic and neurogenic detrusor overactivity,
- Pediatric incontinence, incontinence due to overactive bladder, and incontinence due to neurogenic bladder.
- Anal fissure
- vaginismus To reduce the spasm of the vaginal muscles.
- Movement disorders associated with injury or disease of the central nervous system including trauma, stroke, multiple sclerosis, Parkinson's disease, or cerebral palsy
- Focal dystonias affecting the limbs, face, jaw, or vocal cords
- TMJ pain disorders
- Diabetic neuropathy
- Wound healing
- Excessive salivation
- Vocal cord dysfunction (VCD) including spasmodic dysphonia and tremor
- Reduction of the Masseter muscle for decreasing the apparent size of the lower jaw
- Painful bladder syndrome,
- Detrusor sphincter dys-synergia and benign prostatic hyperplasia
▶ What are the toxin types available in the United States?
In the United States , two BTX serotype preparations available:
BTX-A
Allergan-BOTOX® - Therapeutic (for use with therapeutic indications) and BOTOX® - Cosmetic (for use in the aesthetic indication) Dysport XeominBTX-B
(Myobloc T - Elan Pharmaceuticals ; now owned by Solstice Neurosciences).▶ BTX-A (Allergan, Inc – BOTOX® )
BTX-A (Allergan, Inc- BOTOX® -Therapeutic) is indicated for strabismus, and blepharospasm; an involuntary closure of eyes due to spasm of the muscles surrounding the eyes and the related VII-nerve disorders, cervical dystonia, axillary hyperhidrosis.
The average latency from the time of a BTX-A injection to the onset of improvement is on average, three to five days, and the duration of benefit is usually three to four months. The most frequent side effects, occurring in fewer than 10% of treated patients, include ptosis , blurring of vision, diplopia , tearing, and local haematoma . All these side effects usually resolve in less than two weeks ( Jankovic 2004b) .
▶ BTX-B ( Elan , Inc – Myobloc T )
Myobloc T is a botulinum toxin type-B (BTX-B) preparation and was FDA-approved in 2000 for the treatment of cervical dystonia. BTX-B ( Solcstice-Myobloc T) is available in standard vials containing 2500, 5000, and 10,000U a stable purified neurotoxin type-B complex in liquid formulation and therefore does not require reconstitution.
Data from the manufacturer suggest the product can be used for up to 30 months if refrigerated, or for 9 months if stored at room temperature; the product insert also states that the toxin should be used within 4 hours after initial use, as with all biologics.
Note: Myobloc T was originally granted approval under the ownership and guidance of Élan, Incorporated. In early 2004, Myobloc T was acquired by Solstice Neurosciences)
▶ BTX-A ( Dysport® – Medicis)
Dysport® is a lyophilized (freeze-dried) BTX-A formulation currently available and marketed outside North America .
Dysport® was initially approved in 1991 in various countries outside the United States for spasmodic torticollis , blepharospasm, and hemifacial spasm in adults. A few years later, Dysport® was approved for dynamic equinus foot deformity due to spasticity in ambulant pediatric cerebral palsy patients, two years of age or older, only in hospital specialist centres with appropriately trained personnel ( Ipsen 2004) .
The safety and effectiveness of Dysport® in the treatment of spasmodic torticollis , blepharospasm or hemifacial spasm in children have not been demonstrated. Subsequent approvals were granted for its use to treat spasticity of the lower calf in children with cerebral palsy ( Ipsen 2004) .
Medicis, a cosmetic development company had Dysport® approved for esthetic therapy (frown lines and other facial wrinkles. In addition, clinical trials for Dysport® are underway for the treatment of various forms of motor disorders and muscular spasticity, including treatment of post-stroke spasticity of the upper limbs (phase II studies in Europe) and spasmodic torticollis (phase II studies in the United States).
XEOMIN – MERZ PHARMACEUTICALS 2011
XEOMIN ® is a prescription medicine that is injected into muscles and used:
- • treatment of adults with cervical dystonia, to decrease the severity of abnormal head position and neck pain in both botulinum toxin-naïve and previously treated patients (1.1).
- • treatment of blepharospasm in adults previously treated with onabotulinumtoxinA (Botox®
- • temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients (1.3). (Botox®).
- BOTOX® - Therapeutic Product Insert
- BOTOX® - Cosmetic Product Insert
- Myobloc - Product Insert
- Dysport - Product Insert
▶ What are the FDA-approved indications for BTX?
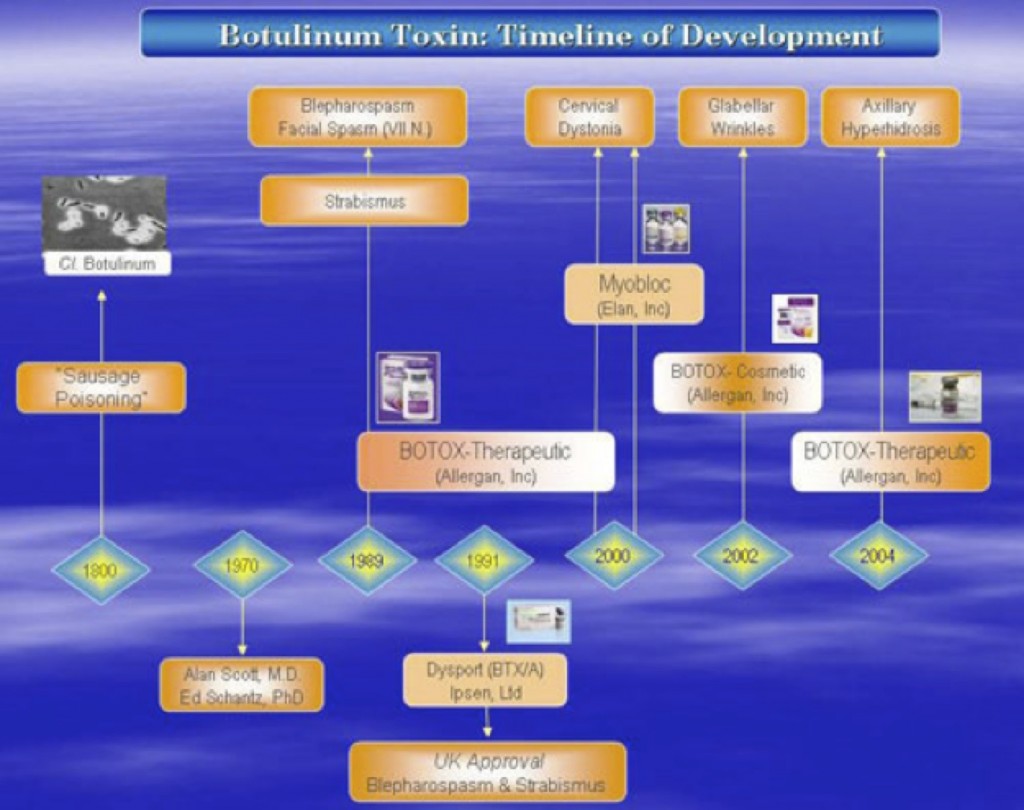
Figure 1 summarizes the FDA-Approved indications for each of the clinical preparations. BTX-A (BOTOX® , Allergan, Inc) is FDA-approved in 1989 for blepharospasm , VII nerve facial disorder , and strabismus ; for cervical dystonia in 2000; BOTOX® – Cosmetic , for – glabellar lines in 2002, and in 2004, for axillary hyperhidrosis . Since the first approval of a BTX formulation (BOTOX® , Allergan, Inc) over 15 years ago, its use has expanded worldwide to over 70 countries, where there are various approvals for over twenty different indications.
A second serotype, BTX-B ( Myobloc T – Elan Pharmaceuticals , was also FDA-approved for cervical dystonia in 2000.
Figure 1: Timeline of FDA-Approvals for Botulinum Toxin
▶ What is Blepharospasm ?
Characterized by abnormal and uncontrollable movement of the eyelids, is in most cases, idiopathic. In contrast, hemifacial spasm is a peripherally induced disorder, caused by an abnormally situated blood vessel that crosses and compresses the seventh craniofacial nerve (Blitzer 2004) . The site of this vascular contact is generally the exit zones of the fifth or seventh nerve, causing a pulsatile compression which is thought to create local demyelination with consequent ephaptic transmission, resulting in paroxysms of hyperactivity causing pain or spasms. ( Coakham 1993) .
▶ What is Facial (Seventh) Nerve Disorder ?
Unlike focal dystonia, which is thought to be of central origin hemifacial spasm is characterized by recurrent, involuntary, synkinetic twitches of the muscles of innervation by the facial nerve (VII), and is typically unilateral. There are rare cases of bilateral hemifacial spasm. In these cases, there is usually an asymmetrical onset and an asynchronous discharge pattern. The twitches may be tonic, clonic , or mixed (Blitzer 2004) .
The prevalence of hemifacial spasm is 14.5 in 100,000 women and 7.4 in 100,000 men. The therapy consists of a choice between oral doses of carbamazepine or valproic acid, an intracranial microvascular decompression of the 7th nerve via a posterior craniotomy, or intramuscular injection of botulinum toxin to reduce or prevent the synchronous spasms. Botulinum toxin injections have the advantage of being simple, safe, and usually indefinitely repeatable in most cases (Blitzer 2004) .
▶ What is Strabismus ?
More commonly known as crossed-eyes, is a vision condition in which a person can not align both eyes simultaneously under normal conditions. One or both of the eyes may turn in, out, up or down. An eye turn may be constant (when the eye turns all of the time) or intermittent (turning only some of the time, such as, under stressful situations or when ill).
▶ What is Cervical Dystonia ?
Botulinum toxin first gained clinical acceptance as a result of marked benefits it produced in patients with dystonia, a neurological disorder dominated by repetitive and patterned contractions of muscles producing abnormal movements and postures.23 While some patients with primary or secondary dystonia improve with levodopa , anticholinergic drugs, baclofen , and muscle relaxants, botulinum toxin injections are now considered the treatment of choice in most patients with focal or segmental dystonia.
Since the initial, double blind, placebo controlled trial of botulinum toxin in patients with cranial-cervical dystonia, including blepharospasm, reported in 1987,24 several controlled and open label studies have confirmed the efficacy and safety of this treatment in a variety of dystonic disorders.23 Moderate or marked improvement has been reported in more than 90% of patients with blepharospasm injected into the orbicularis oculi of upper and lower eyelids. The average latency from the time of the injection to the onset of improvement is three to five days and the duration of benefit is usually three to four months. The most frequent side effects, occurring in fewer than 10% of treated patients, include ptosis , blurring of vision, diplopia , tearing, and local haematoma . All these side effects usually resolve in less than two weeks.
▶ What is Axillary Hyperhidrosis ?
The definition of hyperhidrosis is; Excess sweating beyond what would be
expected for the local environment and is physiologically required by the body ( Stolman 2003) .
Hyperhidrosis (excessive sweating) can be generalized or localized, and secondary or primary; thus, understanding the pattern can help in finding the cause. Generalized hyperhidrosis may be due to an underlying systemic disease or to medication use. Focal hyperhidrosis is often primary (idiopathic) and triggered by emotional stimuli, although it is not generally a psychiatric disease ( Stolman 2003) .
A diagnosis of primary HH is usually based on the patient’s history, typical younger age and visible signs of excessive sweating. Before treatment it is important to objectify focal HH with performing sweat tests such like Minor starch test and/or gravimetry ( Stolman 2003) .
The total number of sweat glands is somewhere between 2 and 4 million and only about 5% are active at the same time, indicating the enormous potential for sweat production. The eccrine sweat gland is a long-branched tubular structure with highly coiled secretory portion and a straight ductular portion. Sweat is produced by clear and dark cells and is a clear hypotonic, odorless fluid. In response to nerve impulses, Acetylcholine (ACh) is released from the presynaptic nerve endings and then binds to postsynaptic cholinergic receptors presumably present in the basolateral membrane of the clear cells. This activates a complex in- and efflux of electrolytes creating the hypotonic sweat (Kreyden and Paul 2004) .
For a long period the therapeutic modalities to treat focal hyperhidrosis (HH) were very limited. With the approval of BTX-A (Allergan, Inc-BOTOX® -Therapeutic in July 2004, clinicians can consider BTX as the therapy of choice for axillary HH after topical treatment with aluminium salts have failed. The amount of successful reports on botulinum toxin (BTX) in the treatment of focal HH brought a change and the interest for this specific disorder grew ( See (Naumann and Jost 2004). for a review on the clinical evidence).
References:
- Blitzer, M. B. A. (2004). Management of hemifacial spasm and facial synkinesis with local injections of botulinum toxin. Oper Tech Otolaryngol Head Neck Surg 15(2): 103-106.
- Coakham , H. (1993). Botulinum toxin for hemifacial s. Br Med J 306(6870): 144.
- Stolman , L. P. (2003). In hyperhidrosis (excess sweating), look for a pattern and cause. Clevel Clin J Med 70(10): 896-898.
- Kreyden, O. P. and Paul, S. E. (2004). Anatomy of the sweat glands, pharmacology of botulinum toxin, and distinctive syndromes associated with hyperhidrosis. Clin Dermatol 22(1): 40-44.
- Naumann, M. and Jost, W. (2004). Botulinum toxin treatment of secretory disorders. Mov Disord 19( Suppl 8): S137-S141.
▶ What is the difference between a vial of BOTOX® Cosmetic and a vial of BOTOX® Therapeutic?
The only difference between the two is in the labeling on the vial and product inserts.
Both products contain a sterile, vacuum-dried purified botulinum toxin type A, produced from fermentation of Hall strain Clostridium purified neurotoxin complex. It is purified from the culture solution by dialysis and a series of acid precipitations to a complex consisting of the neurotoxin, and several accessory proteins. The complex is dissolved in sterile sodium chloride solution containing albumin and is sterile filtered (0.2 microns) prior to filling and vacuum-drying.
Each vial of BOTOX® Cosmetic/BOTOX® Therapeutic contains 100 units (U) of Clostridium botulinum type A neurotoxin complex, 0.5 milligrams of Albumin (Human), and 0.9 milligrams of sodium chloride in a sterile, vacuum-dried form without a preservative.
▶ What is the History behind the development of Therapeutic Botulinum toxins?
- Botulism occurs mostly from eating improperly preserved food. In the eighteenth and nineteenth centuries in Bavaria , botulism was caused by sausages that were preserved with inadequate boiling, smoking, and salting ( Erbguth 1996) . Justinius Kerner collected data on 230 cases of botulism and published two important monographs in 1820 and 1822 ( Erbguth 1996; Kerner 1822) . Kerner gave a remarkably complete and accurate description of clinical botulism: its symptoms, time course, and physical findings, especially that the tear fluid disappears, the pupil dilates, the eye muscles are paralyzed, mucus and saliva secretion is suppressed, the skin is dry, the skeletal muscles and gut are paralyzed, and until the last, cognition is preserved (Scott 2004).
- Finally, Kerner suggested the potential therapeutic use of toxin to block abnormal motor movements, such as chorea, and speculated on its use in other disorders with hypersecretion , for example. However, he stopped there. Kerner was an important romantic poet and a busy medical officer; lacking a university appointment, he went off in these directions at age 37, after remarkably insightful and creative research on botulism (Scott 2004) . His work is summarized in further detail by Smith and more recently by Erbguth and Naumann ( Erbguth 1996; Erbguth and Naumann 1999; Erbguth 2004; Erbguth 1998)
- In 1885 the eminent physiologist Claude Bernard wrote in his classic work Experimental Sciences “Poisons can be employed as a means for the destruction of life or as an agent for the treatment of the sick” (Schantz and Johnson 1997).
- In line with his prediction for medical uses of natural poisons and toxins,160 years after Kerner’s observations and speculation on potential clinical applicability, the idea of therapy with toxin was implemented (Scott 2002) . Clinical development was initiated in the early 1970-1971 by Alan Scott, M.D. an ophthalmologist at Smith- Kettlewell , San Francisco , CA in collaboration with Edward Schantz, PhD. formerly at the University of Wisconsin , Madison , WI . Dr. Scott began to assess injection of various drugs into extraocular muscles as an alternative to surgical treatment for strabismus. Among these was botulinum toxin, which immediately stood out for its long paralytic effect of several weeks, specificity for cholinergic terminals, lack of systemic side effects, and controllable dosage/response relationship.
- In 1989, the FDA licensed botulinum toxin type A as an orphan drug for the treatment of persons who suffer from the involuntary muscle disorders strabismus, blepharospasm, and hemifacial spasm (Schantz and Johnson 1997).
- Clinical acceptance of BTX was gained as a result of marked benefits it produced in patients with dystonia and became the “gold-standard” therapy in the early 1990′s for primary or secondary dystonias ; several years prior to its FDA-approval ( Jankovic 2004a) . This early adoption was due to the significance of a much-needed focally acting treatment to replace oral agents in patients who didn’t get relief and experienced significant systemic related side effects improve with levodopa , anticholinergic drugs, baclofen , and muscle relaxants. BTX injections are now considered the treatment of choice in most patients with focal or segmental dystonia ( Jankovic 2004b).
- Cosmetic use of botulinum toxin, probably its greatest single application, is the creation of Alistair and Jean Carruthers ( Carruthers and Carruthers 1990) . For many years, a few blepharospasm patients injected at intervals of 3 or 4 months around the eyes and upper face would mention as a joke upon return that they were ”back to get the wrinkles out.” The Carruthers ‘ thoughtful and rational application of toxin to selective agonist-antagonistic muscle groups in the face, to lift the brow, flatten folds, is probably overtaken now by less discriminate use.
- It is from widespread use around the face that the beneficial effect of toxin on headache has emerged. Following the Carruther’s cosmetic discovery, in the mid 1990′s, William Binder, M.D., a facial and reconstructive plastic surgeons, had patients he injected for wrinkles report relief of their migraine headache pain following their BTX injection. Dr. Binder, in collaboration with Dr. Blitzer, and Dr. Brin , conducted the first open label study for the use of BTX-A (BOTOX) for migraine pain (Blitzer et al. 1998) . Several controlled trials have followed and is currently in active investigation (See (Ashkenazi and Silberstein 2004) for a recent review.
- Over the years since Dr. Scott’s persistence and ground-breaking research scientists further elucidated that the principal mechanism of BTX is the direct release of muscle tension by its denervating effect at neuromuscular junctions (See (Aoki et al. 2003 ) for further review on the emerging research on the effect of BTX on inflammation and pain.
- In addition to the mechanism of BTX, understanding of the clinical effects, potential for antibody production and new manufacturing and production techniques, led to an improved formulation of BTX-A in 1997 (20-24). Since this formulation was made available, reports of antibodies to botulinum toxin appears to have decreased and the lower protein load may be one of the reasons.
- The initial lot of type A (79-11) licensed to Dr. Scott who branded it as, Oculinum ® by the FDA and later licensed by Allergan who marketed as BOTOX® This original formulation available in the United States, had a relatively low potency, and thus a potential for a higher antigenic protein content (Schantz and Johnson 1993) . The lot used in Europe for BOTOX® (88-4), had a much higher potency and probably fewer antibodies developed (Scott 2004) . Fortunately, both of these original formulations have now been replaced by the higher potency formulation for the Allergan product.
- The pioneering efforts of these original researchers which began as a development for a long-lasting reversible chemodenervation agent for treating blepharospasm and strabismus, has resulted in an agent that has shown significant benefit in numerous disorders, and has brought relief to countless numbers of patients around the world.
- Erbguth , F. J. (1996). Historical note on the therapeutic use of botulinum toxin in neurological disorders. J Neurol Neurosurg Psychiatry 60(2): 151.
- Kerner, J. (1822). Das Fettgift oder die Fettsaure und ihre Wirkungen auf den thierischen Organismus, ein Beytrag zur Untersuchung des in verdorbenen Wursten giftig wirkenden stoffes.(GER). 3.
- Scott, A. B. (2004). Development of botulinum toxin therapy. Dermatol Clin 22(2): 131-133.
- Erbguth , F. J. and Naumann, M. (1999). Historical aspects of botulinum t. Neurology 53(8): 1850-1853.
- Erbguth , F. J. (2004). Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov Disord 19( Suppl 8): S2-S6.
- Erbguth , F. J. (1998). Botulinum toxin, a historical note. Lancet 351(9118): 1820.
- Schantz, E. J. and Johnson, E. A. (1997). Botulinum toxin: the story of its development for the treatment of human disease. Perspect Biol Med 40(3): 317-327.
- Scott, A. B. (2002). The role of botulinum toxin type A in the management of strabismus. 189-195.
- Jankovic , J. (2004a). Dystonia: Medical therapy and botulinum toxin. Adv Neurol 94: 275-286.